BIOMOLECULES
Biomolecules: All the carbon compounds that we get from living tissues.
*The complex organic substances like carbohydrates, proteins etc which combine in a specific manner to produce living systems and maintain it are called biomolecules. The branch of chemistry that deals with the study of chemical reactions that occur in living organisms is called biomolecules.
Micromolecules : Molecules which have molecular weights less than one thousand dalton.
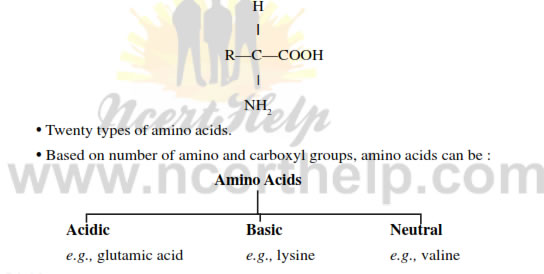
Amino acids: Organic compounds containing an amino group and one carboxyl group (acid group) and both these groups are attached to the same carbon atom called α carbon.
- Twenty types of amino acids occur in proteins.
- Based on number of amino and carboxyl groups, amino acids can be:
- Acidic: e.g. Glutamic acid
- Basic: e.g. Arginine and lysine
- Neutral: e.g. valine, alanine.
Nucleic acids are the polymers of nucleotide made up of carbon, hydrogen, oxygen, nitrogen and phosphorus and which controls the basic functions of the cell. These were first reported by Friedrich Miescher (1871) from the nucleus of pus cell. Altmann called it first time as nucleic acid. Nuclein was renamed nucleic acid by Altman in (1889). They are found in nucleus. They help in transfer of genetic information.
Types of nucleic acids : On the basis of nucleotides i.e., sugars, phosphates and nitrogenous bases, nucleic acids are of two types which are further subdivided. These are DNA (Deoxyribonucleic acid) and RNA (Ribonucleic acid).
(1) DNA (Deoxyribonucleic acids) : Term DNA was given by Zacharis.
(i) Types of DNA : It may be linear or circular in eukaryotes and prokaryotes respectively.
Palindromic DNA : The DNA helix bears nucleotide in a serial arrangement but opposite in two strands.
Repetitive DNA : This type of arrangement is found near centromere of chromosome and is inert in RNA synthesis. The sequence of nitrogenous bases is repeated several times.
Satellite DNA : It may have base pairs up to
(ii) Chargaff’s rule : Quantitatively the ratio of adenine (A) to thymine (T) and guanine (G) to cytosine (C) is equal. i.e., “Purines are always equal to pyrimidine”.
(iii) C value : It is the total amount of DNA in a genome or haploid set of chromosomes.
(iv) Sense and Antisense strand : Out of two DNA strand one which carries genetic information in its cistrons is called sense strand while the other strand does not carry genetic information, therefore, doesn’t produce mRNA. The non-functional DNA strand is called antisense strand.
(v) Heteroduplex DNA : Hybrid DNA formed as a result of recombination is called heteroduplex DNA. It contains mismatched base pair of heterologous base sequence.
X-Ray crystallography study of DNA : It was done by Wilkins. It shows that the two polynucleotide chains of DNA show helical configuration.
Single stranded DNA (ssDNA) : It is single helixed circular. And isolated from bacteriophage
Double helical model of DNA: It is also known as Watson and Crick model.
(2) RNA or Ribonucleic acid : RNA is second type of nucleic acid which is found in nucleus as well as in cytoplasm i.e., mitochondria, plastids, ribosomes etc. They carry the genetic information in some viruses. They are widely distributed in the cell. Genomic RNA was discovered by Franklin and Conrat (1957).
Lipids :
- Water insoluble, containing C, H, O.
- They could be simple fatty acids.
- A fatty acid has a carboxyl group attached to an R group.
- The R group may be a methyl group (-CH3) or ethyl (-C2H5) or higher number of-CH2 group (1 carbon to 19 carbon). E.g. palmitic acid with 19 carbons, arachidonic acid has 20 carbons.
- Fatty acids could be saturated (without double bond) or unsaturated (with one more (c=c) double bond.
- Another example of lipid is glycerol which is trihydroxy propane.
- Many lipids may have both glycerol and fatty acids.
- Fatty acids esterified with glycerol to form mono, di or triglycerides.
- These are also called fats and oils based on the melting points.
- Oils have low melting points (e.g. gingely oil).
- Phospholipids are compound lipids with phosphorus and a phosphorylated organic compound. They are found in the cell membrane. e.g., Lecithin.
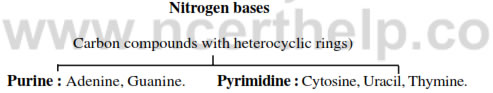
Nitrogen bases :
- Carbon compounds with heterocyclic rings.
- Purine: Adenine, Guanine.
- Pyrimidine: Cytosine, Uracil, Thymine.
- Nucleoside: Nitrogenous base + Sugar e.g., Adenosine, guanosine, thymidine Uridine and cytidine
- Nucleotide: Nitrogenous base + Sugar + Phosphate group. e.g., Adenylic acid, thymidylic acid, guanylic acid, uridylic acid and cytidylic acid.
- Nucleic acid: Polymer of nucleotides - DNA and RNA.
PRIMARY AND SECONDARY METABOLITES :
- Primary metabolites :
- Have identified function.
- Play known roles in physiological function.
- Carbohydrates, amino acids, fats and oils, nitrogen bases are the example of primary metabolites.
- Secondary metabolites :
- Have no definite function.
- Have no direct role in normal physiology.
- Alkaloid, favonoides, rubber, essential oils, antibiotics, coloured pigments. Scents, gums, spices are some example.
- Biomacromolecules : Biomolecules with molecular weights in the range of ten thousand daltons and above; found in acid insoluble fraction.
- Lipids are not strictly macromolecules as their molecular weights do not exceed 800 Da but form a part of the acid insoluble pool.
Macromolecules are polymerisation product of micromolecuels, have high molecular weight and low solubility. They include mainly polysaccharide, protein and nucleic acids.
(1) Polysaccharide : They are branched or unbranched polymers of monosaccharides jointed by glycosidic bond. Their general formula is Polysaccharides are amorphous, tasteless and insoluble or only slightly soluble in water and can be easily hydrolysed to monosaccharide units.
Types of polysaccharides
(i) On the basis of structure
Homopolysaccharides : These are made by polymerisation of single kind of monosaccharides. e.g., starch, cellulose, glycogen, etc.
Heteropolysaccharide : These are made by condensation of two or more kinds of monosaccharides. e.g., chitin, pectin, etc.
(ii) On the basis of functions
Food storage polysaccharides : They serve as reserve food. e.g., starch and glycogen.
Structural polysaccharides : These take part in structural framework of cell wall e.g., chitin and cellulose.
Description of some polysaccharides
Glycogen : It is a branched polymer of glucose and contain 30,000 glucose units. It is also called animal starch. It is also found as storage product in blue green algae, slime moulds, fungi and bacteria. It is a non-reducing sugar and gives red colour with iodine. In glycogen, glucose molecule are linked by glycosidic linkage in straight part and linkage in the branching part glycogen has branch points about every glucose units.
Starch : Starch is formed in photosynthesis and function as energy storing substance. It is found abundantly in rice, wheat, legumes, potato (oval and ecentric shaped), banana, etc. Starch is of two types. Straight chain polysaccharides known as amylose and branched chain as amylopectin. Both composed of glucose units jointed by linkage and linkage. It is insoluble in water and gives blue colour when treated with iodine.
Inulin : Also called “dahlia starch”(found in roots). It has unbranched chain of 30 – 35 fructose units linked by glycosidic linkage between 1 and 2 of carbon atom of D– fructose unit.
Cellulose : An important constituent of cell wall made up of unbranched chain of glucose units linked by 1 – 4 glycosidic linkage. It is fibrous, rigid and insoluble in water. It doesn’t give any colour when treated with iodine. It is a most abundant polysaccharide.
Chitin : It is a polyglycol consisting of acetylglucosamine units connected with glycosidic linkage. Mostly it is found in hard exoskeleton of insects and crustaceans and some times in fungal cell wall. Second most abundant carbohydrate. It is a most abundant heteropolysaccharide.
Agar-Agar : It is a galactan, consisting of both D and L galactose and it is used to prepare bacterial cultures. It is also used as luxative and obtained from cell wall of red algae e.g., Gracilaria, Gelidium etc.
Pectin : It is a cell wall material in collenchyma tissue may also be found in fruit pulps, rind of citrus fruits etc. It is water soluble and can undergo sol
gel transformation. It contain arabinose, galactose and galacturonic acid.
Neutral sugars : It is found associated with cellulose in cell wall. The common sugars in hemicellulose are
xylose, arabinose,
galactose,
mannose and
glucusonic acid. e.g., hemicellulose.
Gum : It secreted by higher plants after injury or pathogenic attacks. It is viscous and seals the wound. It involves sugars like L-arabinose,
galactose, glucusonic acid. e.g., gum arabic.
(2) Mucopolysaccharides : These are gelatinous substance, containing amino sugars, uronic acid, etc. All slimy substances of plant are mucopolysaccharide. e.g., hyaluronic acid, vitreous humour, chondridine sulphate, heparin, husk of isabgol and mucilage also.
Glycoproteins : They include some plasmaprotein and blood group substances. They doesn’t contain uronic acid.
Murein : It is a peptidoglycan, linked to short chains of peptides. It is constituent of cell wall of bacteria and blue green algae.
Functions
(i) Cellulose pectin and chitin are constituents in cell wall of higher plants but peptidoglycan in the cell wall of prokaryotes.
(ii) They are reserve food material and form protective covering.
(iii) Fibres are obtained used in making cloth and rope.
(iv) Nitrocellulose and trinitrate cellulose (gun-cotton) used as explosive.
(3) Protein : The word protein was coined by Berzelius in 1838 and was used by G. J. Mulder first time 1840. 15% of protoplasm is made up of protein.
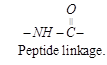
Average proteins contain 16% nitrogen,
carbon, oxygen hydrogen 7% and sulphur
Iron, phosphorous, copper, calcium, and iodine are also present in small quantity.
Structure of proteins : It is due to different rearrangement of amino acids. When carboxyl group
of one amino acid binded with amino group of another amino acid the bond is called peptide bond.
(i) Primary structure : The primary structure is the covalent connections of a protein. It refers to linear sequence, number and nature of amino acids bonded together with peptide bonds only. e.g., ribonuclease, insulin, myoglobin and lysozyme.
(ii) Secondary structure : The folding of a linear polypeptide chain into specific coiled structure
helix) is called secondary structure. e.g., fur, keratin of hair claws, and feathers.
(iii) Tertiary structure : The arrangement and interconnection of proteins into specific loops and bends is called tertiary structure of proteins. It is found in e.g., globular proteins.
(iv) Quarternary structure : It is shown by protein containing more than one peptide chain. The protein consists of identical units. It is known as homologous quarternary structure e.g., lactic dehydrogenase. If the units are dissimilar, it is called as heterogeneous quarternary structure e.g., haemoglobin.
Classification of proteins : Proteins are classified on the basis of their shape, constitution and function.
On the basis of shape
Fibrous protein/Scleroprotein : Insoluble in water. Animal protein resistant to proteolytic enzyme is spirally coiled thread like structure form fibres. e.g., collagen (in connective tissue), actin and myosin, keratin in hairs, claws, feathers, etc.
Globular proteins : Soluble in water. Polypeptides coiled about themselves to form oval or spherical molecules e.g., albumin insulin hormones like ACTH, oxytosin, etc.
On the basis of constituents
Simple proteins : The proteins which are made up of amino acids only. e.g., albumins, globulins, prolamines, glutelins, histones, etc.
Conjugated proteins : These are complex proteins combined with characterstic non–amino acid substance called as prosthetic group. These are of following types :
(i) Nucleoproteins : Combination of protein and nucleic acids, found in chromosomes and ribosomes. e.g., deoxyribonucleoproteins, ribonucleoproteins, etc.
(ii) Mucoproteins : These are combined with large amount (more than 4%) of carbohydrates e.g., mucin.
(iii) Glycoproteins : In this, carbohydrate content is less (about 2 – 3%) e.g., immunoglobulins or antibiotics.
(iv) Chromoproteins : These are compounds of protein and coloured pigments. e.g., haemoglobin, cytochrome, etc.
(v) Lipoproteins : These are water soluble proteins and contain lipids. e.g., cholesterol and serum lipoproteins.
(vi) Metalloprotein : These are metal binding proteins, AB1–globin known as transferring is capable of combining with iron, zinc and copper e.g., chlorophyll.
(vii) Phosphoprotein : They composed of protein and phosphate e.g., casein (milk) and vitellin (egg).
Derived proteins : When proteins are hydrolysed by acids, alkalies or enzymes, the degredation products obtained from them are called derived proteins.
On the basis of nature of molecules
Acidic proteins : They exist as anion and include acidic amino acids. e.g., blood groups.
Basic proteins : They exist as cations and rich in basic amino acids e.g., lysine, arginine etc.
Function of Proteins
(i) Proteins occur as food reserves as glutelin, globulin casein in milk.
(ii) Proteins are coagulated in solutions, alkaline to the isoelectric pH by positive ions such as
etc. Casein resum globulin 5.4, pepsin 2.7, lysozyme 11.0 etc.
(iii) Proteins are the most diverse molecule on the earth.
(iv) They are biological buffers.
(v) Monelin is the sweetest substance obtained from African berry (2000 time sweeter than sucrose).
(vi) Most abundant protein on earth is RUBP.
(vii) Myosin is structural as well as enzymatic protein (ATPase).
Proteins :
- Are polymers of amino acids linked by peptide bond.
- Is a heteropolymer not homopolymer.
- Essential amino acids: those can’t be synthesized in our body, have to be supplied through our diet.
- Non-essential amino acids: our body can synthesize it from other sources.
- Collagen is the most abundant protein in animal.
- Ribulose bisphosphate Carboxylase-Oxygenase (RUBISCO) is most abundant protein in the whole biosphere.
POLYSACCHARIDES :
- Acid soluble pillet also has polysaccharides as another class of macromolecules.
- Polysaccharides are the long chain of sugars.
- Cellulose is homopolymer containing only glucose units.
- Starch is a variant of homopolymer of glucose which store energy.
- Glycogen is another homopolymer found in animal.
- Inulin is a polymer of fructose.
- In a polysaccharide chain the right end is called reducing end and left end is non-reducing end.
- Starch form helical secondary structure.
- Starch can hold Iodine (I2) molecule in its helical portion hence gives blue colour.
- Cellulose dose not contain complex helices and hence cannot hold Iodine (I2) and not give blue colour.
- Complex sugars have amino-sugar as building blocks. (Glucosamine, N-acetyl galactosamine.)
- Exoskeleton of arthropods made of complex sugar called chitin.
- Complex polysaccharides are heteropolymer.
STRUCTURE OF PROTEINS :
- Primary structure: Is found in the form of linear sequence of amino acids. First amino acid is called N-terminal amino acid and last amino acid is called C-terminal amino acid.
- Secondary structure: Polypeptide chain undergoes folding or coiling which is stabilized by hydrogen bonding. Right handed helices are observed. e.g., fibrous protein in hair nails.
- Tertiary structure: Long protein chain is folded upon itself like a hollow woolen ball. Gives a 3-dimensional view of protein, e.g., myosin.
- Quaternary structure: Two or more polypeptides with their folding and coiling are arranged with respect to each other. e.g., Human haemoglobin molecule has 4 peptide chains - 2α and 2β subunits.
NATURE OF BOND LINKING MONOMERS IN A POLYMER :
- Peptide bondb : Formed between the carboxyl (-COOH) group of one amino acid and the amino (-NH2) group of the next amino acid with the elimination of water moiety.
- Glycosidic bondb :
- Individual monosaccharides linked with each other to form polysaccharides.
- This bond is also formed by dehydration.
- Formed between two carbon atoms of two adjacent monosaccharides.
- Phosphodiester bondbb :
- In a nucleic acid a phosphate moiety links the 3’-carbon of one sugar one nucleotide to the 5’-carbon of the sugar of the succeeding nucleotide.
- The bond between the phosphate and hydroxyl group of sugar is an ester bond.
- There is one such ester bond on either side, it is called Phosphodiester bond.
- Anabolic pathways: Lead to formation of more complex molecules from a simpler molecules with the consumption of energy. e.g., Protein from amino acids.
- Catabolic pathway: Lead to formation of simpler molecule from a complex molecule. e.g., Glucose → Lactic Acid.
ENZYMES :
- Are biocatalysts.
- Almost all enzymes are proteins.
- Ribozyme - Nucleic acids that behave like enzymes.
- Has primary, secondary and tertiary structure.
- Active site of an enzyme is a crevice or pocket into which substrate fits.
- Enzymes get damaged at high temperatures.
- Enzymes isolated from thermophilic organisms (live under high temperatures) are thermostable.
- Enzymes accelerate the reactions many folds.
- Enzymes lower the activation energy of reactions.
- The chemicals on which the enzyme acts called substrates.
- Enzyme converts substrates into products.
Nature of enzyme action :
- The substrate binds to the active site of the enzyme, fitting into the active site.
- The binding of the substrate induces the enzymes to alter its shape, fitting more tightly around the substrate.
- Active site now breaks the chemical bond of the substrate and enzyme-product complex is formed.
- The enzyme releases the product.
Factors affecting enzyme activity :
- Temperature :
- Show highest activity at optimum temperature.
- Activity declines above and below the optimum value.
- pH :
- Enzymes function in a narrow range of pH.
- Highest activity at optimum pH.
- Concentration of substrate :
- The velocity of enzymatic reaction rises with increase in substrate concentration till it reaches maximum velocity (V max). Further increase of substrate does not increase the rate of reaction as no free enzyme molecules are available to find with additional substrate.
- Enzyme inhibition: When the binding of a chemical shuts off enzyme activity, the process is called inhibition and chemical is called inhibitor.
- Competitive inhibition: Inhibitor closely resembles the substrate in its molecular structure and inhibits the enzyme activity. E.g., inhibition of succinic dehydrogenase by malonate.
Classification of enzymes :
- Oxidoreductase/dehydrogenases: Catalyse oxidoreduction between 2 substrates.
- Transferases: Catalyse transfer of a group between a pair of substrates.
- Hydrolases: Catalyse hydrolysis of ester, ether, peptide, glycosidic, C-C, P-N bonds.
- Lyases: Catalyse removal of groups from substrates by mechanisms other than hydrolysis leaving a double bond in the product.
- Isomerases: Catalyse inter-conversion of optical, geometric or positional isomers.
- Ligases: Catalyse linking together of 2 compounds.
Cofactors :
- Non-protein constituents found to the enzyme to make it catalytically active.
- Protein portion of enzyme is called apoenzyme.
- Prosthetic groups: Are organic compounds tightly bound to apoenzyme. E.g., haem in peroxydase and catalase.
- Co-enzymes: Organic compounds which loosely bind with enzyme. E.g., NAD, NADP.
- Metal ions: Required for enzyme activity. Form coordination bond with side chains at active site and with substrate. E.g., zinc is a co-factor for enzyme enters stomach?
Glycerol : A simple lipid, is trihydroxy propane.
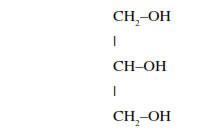
• Some lipids have fatty acids esterified with glycerol.
• They can be monoglycerides, diglycerides and triglycerides.
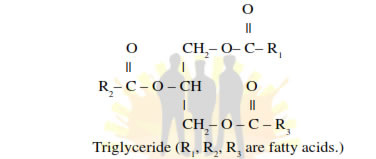
• Phospholipids are compound lipids with phosphorus and a phosphory- lated organic compound e.g., Lecithin .



0 Comments:
Post a Comment